ESTABLISHING ORDER IN THE PERIODICAL TABLE OF ELEMENTS - PMM 407
MOSELEY, H.G.J.
The High-Frequency Spectra of the Elements, I-II.
London, 1913 & 1914.
8vo. 2 volumes, uniformly bound with the original wrappers in recent full blue cloth. In "The London, Edinburgh and Dublin Philosophical Magazine", Sixth Series, Vol. 26, no. 156, December 1913 & vol. 27, no. 160, April 1914. Lower part of index (pp. 1059-1064) in vol 26 with horisontal repair to lower part, affecting last three line (but legible). A fine and clean set. Moseley's papers: pp. 703-13; pp. 1024-1034. [Entire issues: pp. 937-1064; pp. 541-756].
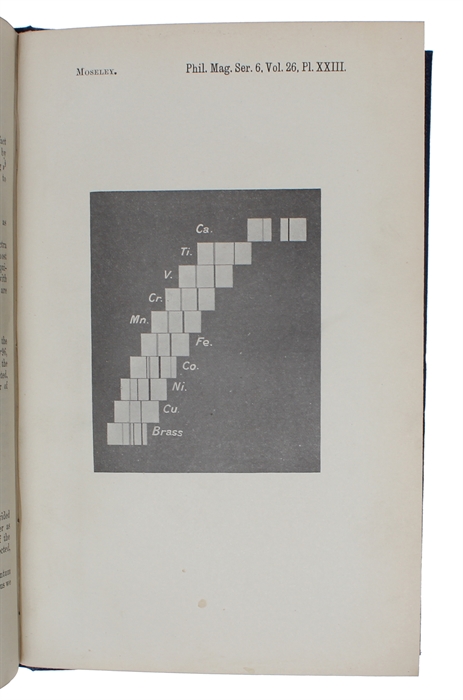
First edition of these groundbreaking papers, in which the arrangement of the elements in the periodic table was based on the atomic number and which thus placed the atomic table on a firm scientific foundation. "Moseley, working under Rutherford at Manchester, used the method of X-ray spectroscopy devised by the Braggs to calculate variations in the wave-lenght of the rays emitted by each element. These he was able to arrange in a series according to the nuclear charge of the element. Thus if the nuclear charge of hydrogen is 1, in helium it is 2, in lithium 3, and so on by regular progression to uranium as 92. These figures Moseley called atomic numbers.he pointed out that they also represented a corresponding increase in extra-nuclear electrons and that it is the number and arrangement of these electrons rather than the atomic weight that determines the properties of an element. It was now possible to base the periodical table on a firm foundation, and to state with confidence that the number of elements up to uranium is limited to 92. When Moseley'stable was completed, six atomic numbers had no corresponding elements; but Moseley himself was able to predict the nature of four of the missing elements."(PMM 407). “In a very short time, Moseley produced the first of his two famous papers in which he showed the spectra of K radiation of ten different substances … Moseley arranged the spectra, one below the other in a step-like fashion, in such a way that a given wavelength was in the same position for all spectra. It then became clear by simple inspection of this ‘step ladder’ that the spectrum of K radiation of each element contains two strong lines (which Moseley called Ka (for the longer wavelength) and Kß (for the shorter) and that this pair of lines moves to shorter and shorter wavelengths in a monotonic fashion if one moves step by step from calcium to zinc Moseley's work made it clear once and for all that indeed the position number in the Periodic Table is equal to the number Z of positive elementary charges in the nucleus of an atom. It also showed that Z is more important for the spectroscopic and chemical properties of an atom than the atomic mass number A. This is evident in the case of the elements cobalt (Z = 27, A = 58.9) and nickel (Z = 28, A = 58.7), where even the order in A differs from that in Z.” (Brandt, The Harvest of a Century: Discoveries in Modern Physics in 100 Episodes)
PMM 407
Evans 62
Norman 1599
Order-nr.: 60037