FOUNDATION OF ELECTRO-CHEMISTRY - OFF-PRINT, PRESENTATION-COPY
DAVY, HUMPHRY.
The Bakerian Lecture, on some chemical Agencies of Electricity. Read November 20, 1806.
London, Philosophical Transactions, 1807.
4to. Bound to style in recent plain blue wrappers. Offprint, with the separate printed title-page, from "Philosophical Transactions" 1807 - Part I. With author's presentation to title-page: "From the Author". Occassional brownspotting throughout and a small tear, not affecting text, to lower margin of B4. (2), 56 pp. + 1 plate.
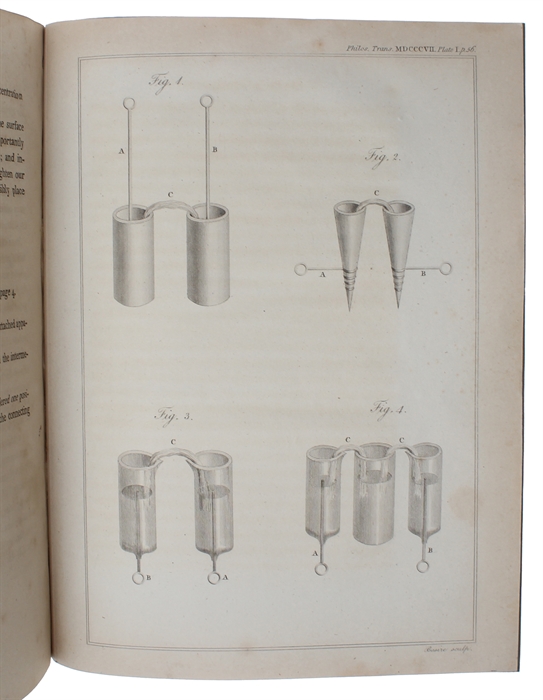
The exceedingly rare offprint, inscribed presentation copy, of Davy’s milestone paper in which he shows that electricity is capable of decomposing the most stable elements. The paper was central to any chemical affinity theory in the first half of the nineteenth century and Berzelius, one of the founders of modern chemistry, considered it "one of the best memoirs which has ever enriched the theory of chemistry”. Davy early concluded that the production of electricity in simple electrolytic cells resulted from chemical action and that chemical combination occurred between substances of opposite charge. He therefore reasoned that electrolysis, the interactions of electric currents with chemical compounds, offered the most likely means of decomposing all substances to their elements. “These views were explained in 1806 in his lecture “On Some Chemical Agencies of Electricity,” for which, despite the fact that England and France were at war, he received the Napoleon Prize from the Institut de France (1807). This work led directly to the isolation of sodium and potassium from their compounds (1807) and of the alkaline-earth metals magnesium, calcium, strontium, and barium from their compounds (1808).” (Britannica). "Humphry Davy was one of the most brilliant chemists of the early nineteenth century. His early study of nitrous oxide brought him his first reputation, but his later and most importent investigations were devoted to electrochemistry. Following Galvani's experiments and the discovery of the voltaic pile, interest in galvanic electricity had become widespread. The first electrolysis by means of the pile was carried out in 1800 by Nicholson and Carisle, who obtained oxygen and hydrogen from water. Davy began to examine the chemical effects of electricity in 1800, and his numerous discoveries were presented in his Bakerian lecture to the Royal Society on November 20, 1806 (the paper offered here). His experiments, along the lines stated in this paper, lead to his discoveries of potassum and sodium in 1807 and the year after to barium, calcium and boron.” (A Source Book in Chemistry p. 243). Sparrow: Milestones of Science No 52.
Wheeler Gift: 2511.
(PMM 255)
Order-nr.: 60110